
enterprise:Xinxiang qiangsheng medical equipment co. LTD
cellphone:18568235005fax:0373-8962789
URL : www.hnqiangsheng.com
Address: dingluan town industrial zone, changyuan county, xinxiang city, henan province
Welcome to xinxiang qiangsheng medical equipment co., LTD.!
[product name] disposable sterile production bag
Structure and composition of the disposable use asepsis production package 】 by the basic configuration in disposable, disposable medical pad, and use configuration disposable surgical masks, disposable medical hats, disposable gown, legs, disposable towels, gauze bandages treatment (umbilical cord volume), umbilical cord, examination gloves (PE), disposable bag cloth, medical cotton swabs, medical gauze piece, disposable drapes, non absorbable surgical sutures, medical gauze pad, tray, disposable sterilized rubber surgical gloves, disposable rubber examination gloves, iodine volts of cotton ball, shoe-covers, umbilical cord cap.
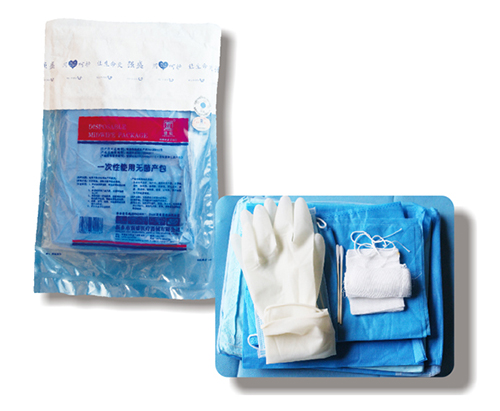
[model specification] maternity type.
1) the package shall be well sealed, seamless and free from holes.
2) all parts in the bag should be arranged neatly and the surface should be clean without stains, impurities and damage.
3) sewn articles should be firmly sewn, uniform needle code, straight, no wrinkles, no jumping needle, thread opening, seam leakage and other phenomena.
4) folded items should be neatly folded.
5) package in producing basic configuration in disposable, disposable medical pad, and use configuration disposable surgical masks, disposable medical hats, disposable gown, legs, disposable towels, gauze bandages treatment (umbilical cord volume), umbilical cord, examination gloves (PE), disposable bag cloth, medical cotton swabs, medical gauze piece, disposable drapes, non absorbable surgical sutures, medical gauze pad, tray, disposable sterilized rubber surgical gloves, disposable rubber examination gloves, iodine volts cotton ball, shoe covers, umbilical cord cap should be chosen with medical equipment product registration certificate of the product, and have a warranty.
6) the products should be sterile.
7) the production package is sterilized by ethylene oxide, and the residual amount of ethylene oxide should be no more than 10 microns /g.
[scope of application] it is suitable for the use of obstetrics and gynecology in medical institutions.
[usage] open the package and use it in accordance with the routine medical operation.
[production date] see the sealing of the packaging bag or the qualification certificate in the bag.
[sterilization date] see sterilization certificate.
[expiration date] see the sealing of the packaging bag or the certificate of conformity in the bag.
[matters needing attention, warnings and tips]
1) check the package before use. Do not use it if the package is damaged.
2) this product has been sterilized by ethylene oxide, and the sterile period is two years.
3) this product is only for one-time use. After use, it is non-recyclable medical waste. Please treat it as such waste.
[storage method] it should be stored in a ventilated, clean and cool place with relative humidity no more than 80%.Avoid with fire source, toxic, volatile
Contact with corrosive substances.
Contraindications: none.
Attachment: the interpretation of the graphics, symbols, abbreviations and other contents of the medical devices used in this product
One-time use reading instructions batch number: no. : no. : no. : no. : no. : no. : no. : no. : no. : no. : no. : no.
[address] guandong, dingluan town, changyuan county
[after-sales service unit] xinxiang qiangsheng medical equipment co., LTD
[address] guandong, dingluan town, changyuan county
[production address] kanto, dingluan town, changyuan county
[fax: 0373-8962222] 0373-8962789
h.nqs@163.com
[date of instruction] July 9, 2017